Nanodevices for the spatial control of immunoreceptors
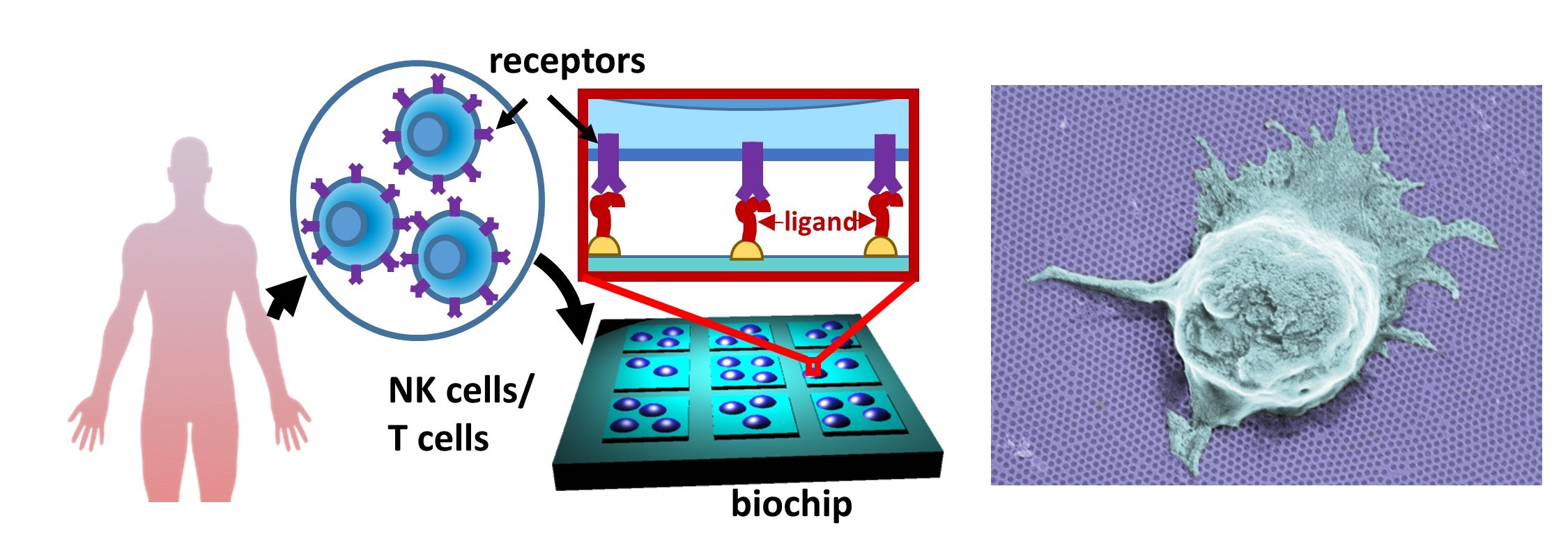
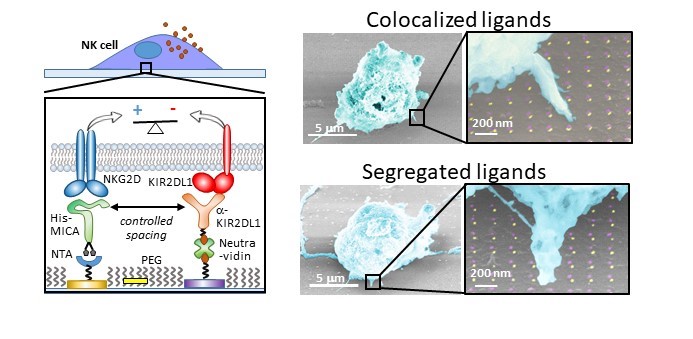
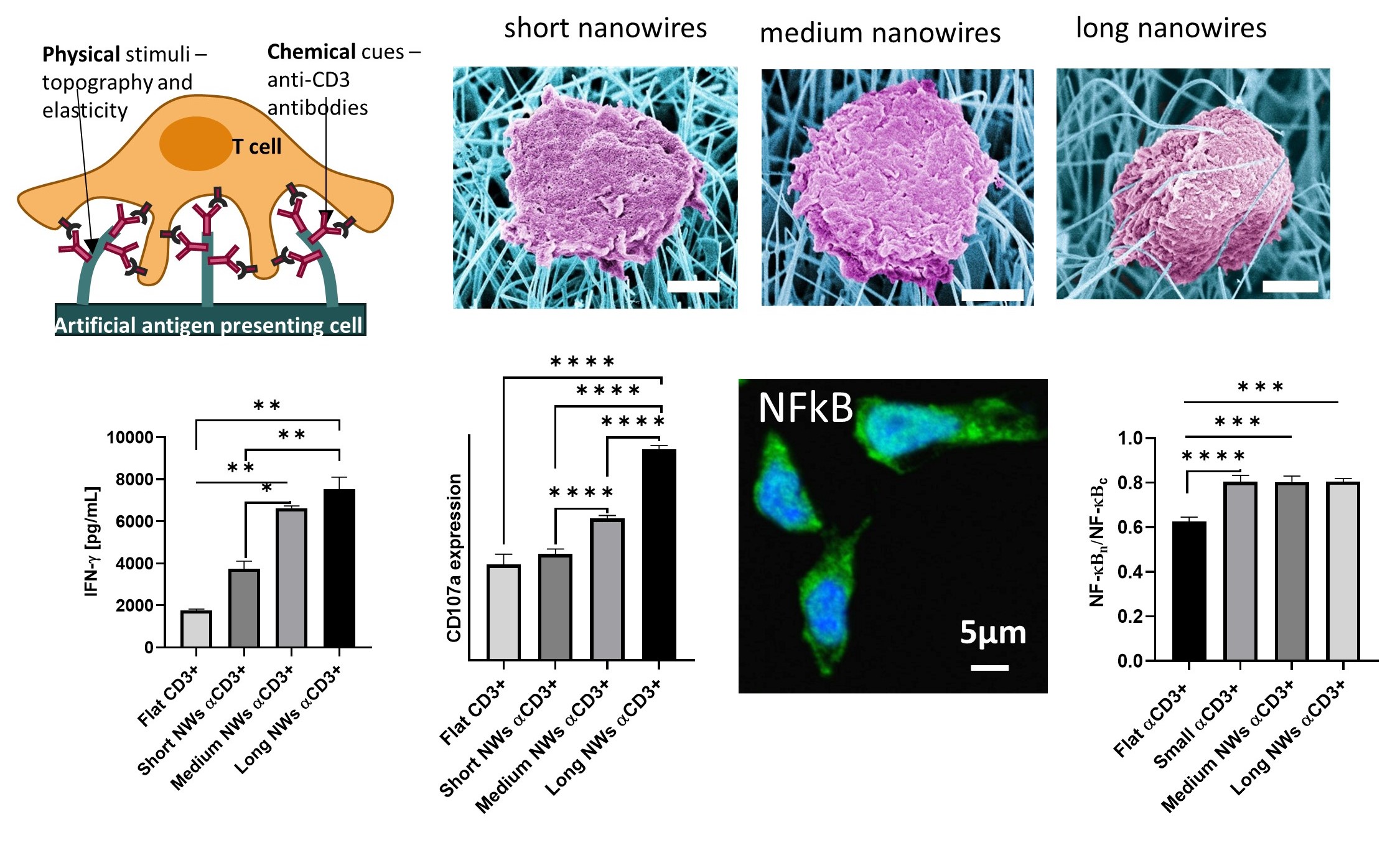
T cells and Natural Killer (NK) cells serve as the sentinels of our immune system, with their cytotoxic activity governed by a rich repertoire of activating, costimulatory, and inhibitory receptors. Furthermore, this activity is regulated by physical environmental cues, such as the spatial nanoscale organization of these receptors, the elasticity of the target cells, and the morphology of the interface between the target and T/NK cells. We employ nanotechnological approaches to understand how these cues, individually and collectively, regulate the response of T/NK cells. Leveraging cutting-edge nanolithography, we engineer nanochip devices patterned with molecular-scale arrays of ligands recognized by the activating receptors of T and NK cells. These devices serve as artificial antigen-presenting cells encoding the spatial arrangement of specific cell receptors. The ligands on these devices can be arranged arbitrarily, either in nanoscale clusters that mimic naturally occurring clusters in the T/NK cell target interface or as a mosaic of individual molecules. To comprehend the impact of spatial distribution on signaling integration between various receptors—such as those with activating and inhibitory functionalities—sophisticated fabrication approaches are required to precisely position different ligands on the same chip surface. We recently demonstrated such control at the molecular level through nanolithographic patterning of metallic nanodimers combined with trigonal site-selective functionalization of both the nanodimers and their background. Using this approach, we investigated the effect of spacing between activating and inhibitory receptors in NK cells. Our findings revealed that signaling integration does not solely depend on spatial proximity but also on the perpendicular dimension to the membrane of the two molecules.
Read more
* equal contribution, Science Adv. 7, eabc1640 (2021)Y
Nanomaterials to study lymphocyte mechanosensing
Utilizing such arrays, we presented the first direct evidence that NK cells possess mechanosensory capabilities, employing mechanical forces to distinguish between healthy and stressed or infected cells. Subsequently, we engineered a mechanostimulating platform based on nanowires with tunable length, providing T cells and NK cells with precisely measured elastic cues. Our studies revealed that these cues not only mechanostimulate these cells but also dictate the extent of their response.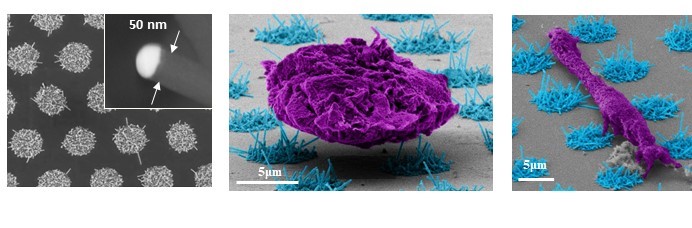 Mechanical forces also play a critical role in the activity of T cells and NK cells, yet their detection and study pose challenges as they primarily operate at the nanometric length scale. We employ both bottom-up approaches to produce controlled arrays of nanowires, which can then be functionalized with activating ligands to provide distinct mechanical, chemical, and topographic stimuli for cytotoxic lymphocytes.
Mechanical forces also play a critical role in the activity of T cells and NK cells, yet their detection and study pose challenges as they primarily operate at the nanometric length scale. We employ both bottom-up approaches to produce controlled arrays of nanowires, which can then be functionalized with activating ligands to provide distinct mechanical, chemical, and topographic stimuli for cytotoxic lymphocytes. Read more
Adv. Mater. 31,1805954 (2019) * equal contribution
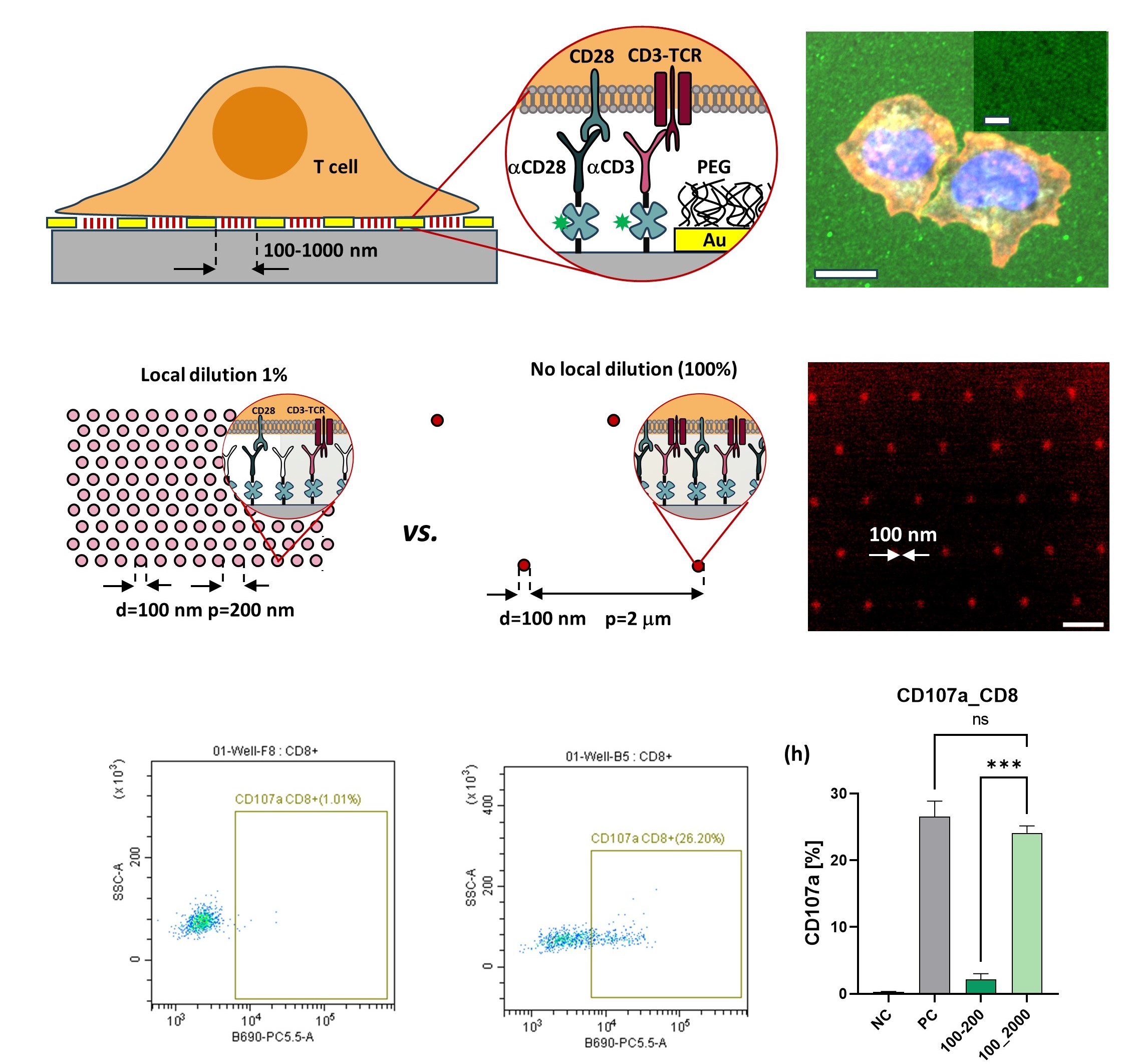
Novel nanomaterials for cancer immunotherapy
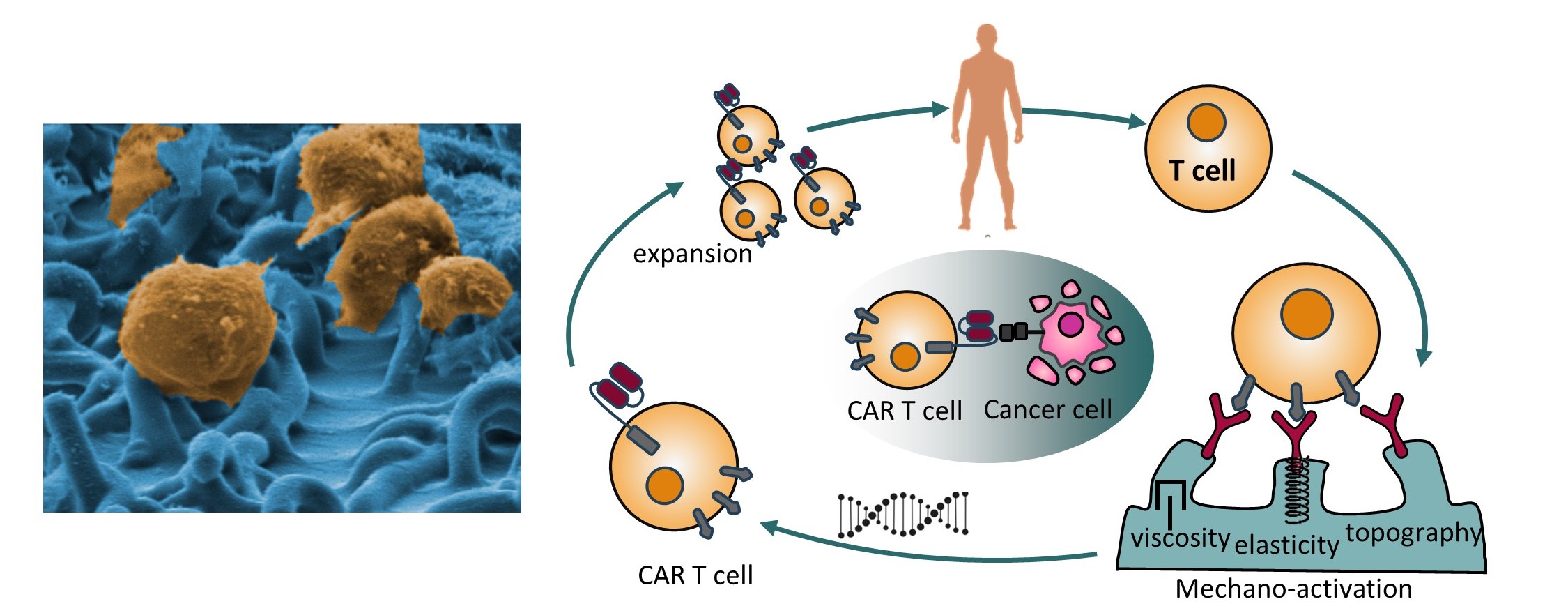
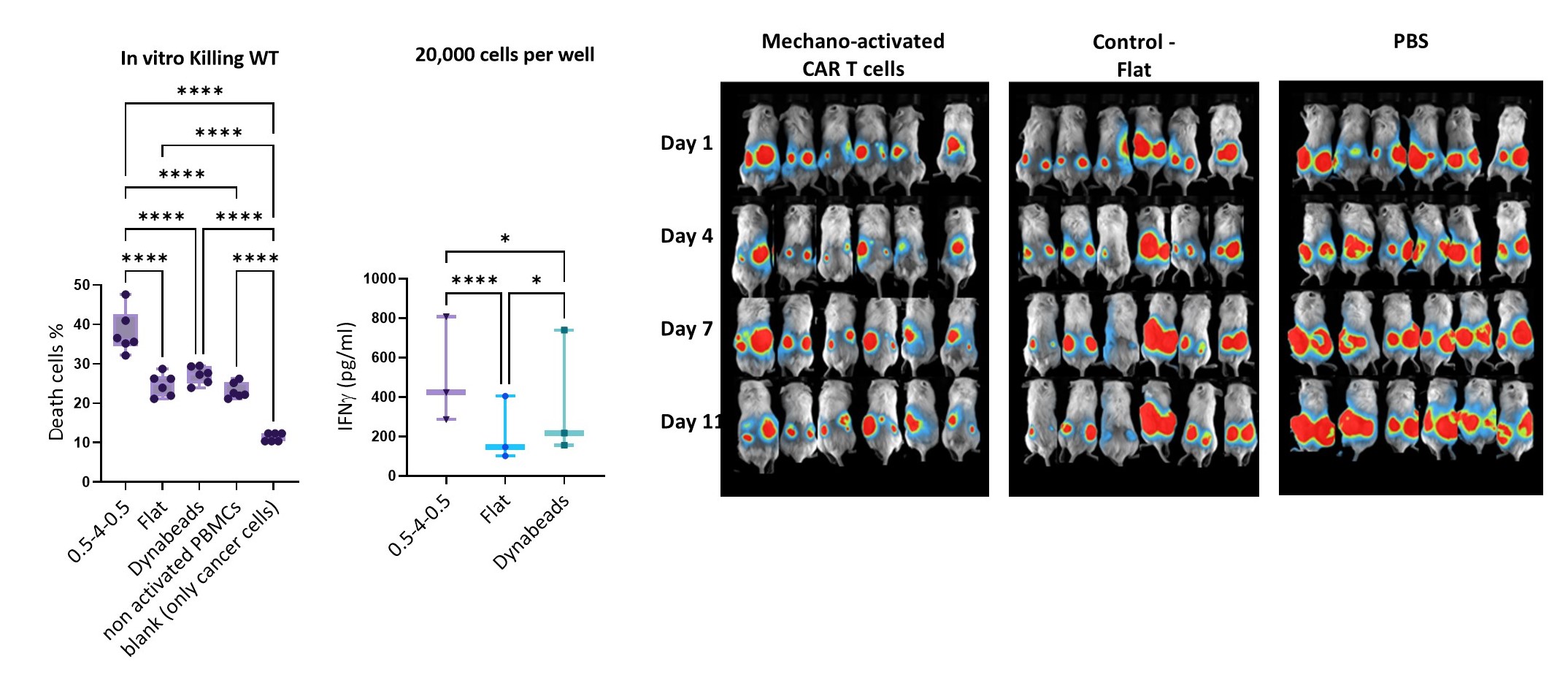
Adoptive immunotherapy, based on genetically modified T cells expressing chimeric antigen receptors (CARs), has revolutionized cancer treatment through cellular immunotherapy. This therapy involves isolating T cells from the patient, activating them with artificial target cells—typically in the form of microbeads covered with activating and costimulatory antigens—genetically modifying them with CARs, expanding their numbers, and reintroducing the CAR-expressing cells into the patient’s body, where they effectively combat cancer. However, bead-based activation often yields highly ineffective, exhausted CAR T cells, thus limiting their therapeutic efficacy. To address this, we have developed novel mechanostimulating T cell activation platforms aimed at generating CAR T cells with enhanced immunotherapeutic potency. Our platform incorporates rationally designed topography and elasticity into the antibody-carrying surfaces. Specifically, we have demonstrated that 3D micropatterns of elastic materials functionalized with antibodies induce robust activation and proliferation of T cells while minimizing exhaustion. We have integrated this platform into the production of CAR T cells. The resulting CAR T cells exhibited superior ex-vivo and in-vivo (in mouse models) antitumor activity compared to CAR T cells produced using standard technology. These findings represent a groundbreaking demonstration that the elasticity and microstructure of T cell activating surfaces can be harnessed to augment the efficiency of CAR T cell immunotherapy, paving the way for the development of new biomaterials for this revolutionary cancer treatment. Current research efforts are focused on the development of mechanostimulating platforms for the personalized production of immunotherapeutic CAR T cells tailored to individual patients.Read more
Self-assembly based nanofabrication
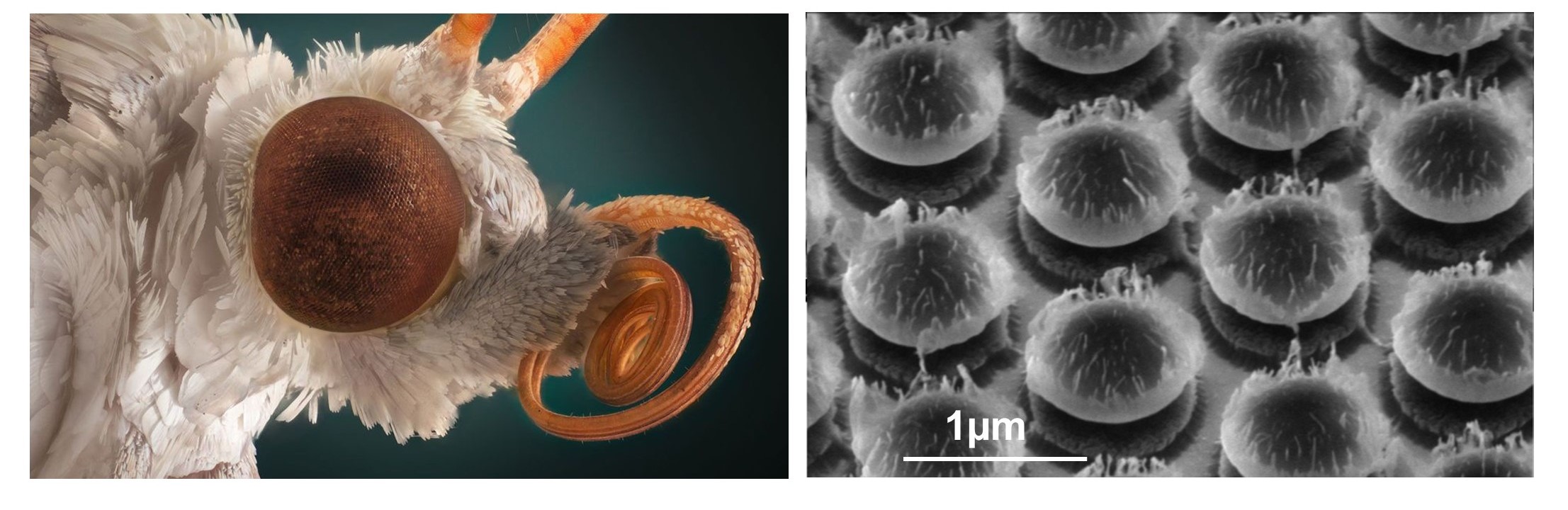
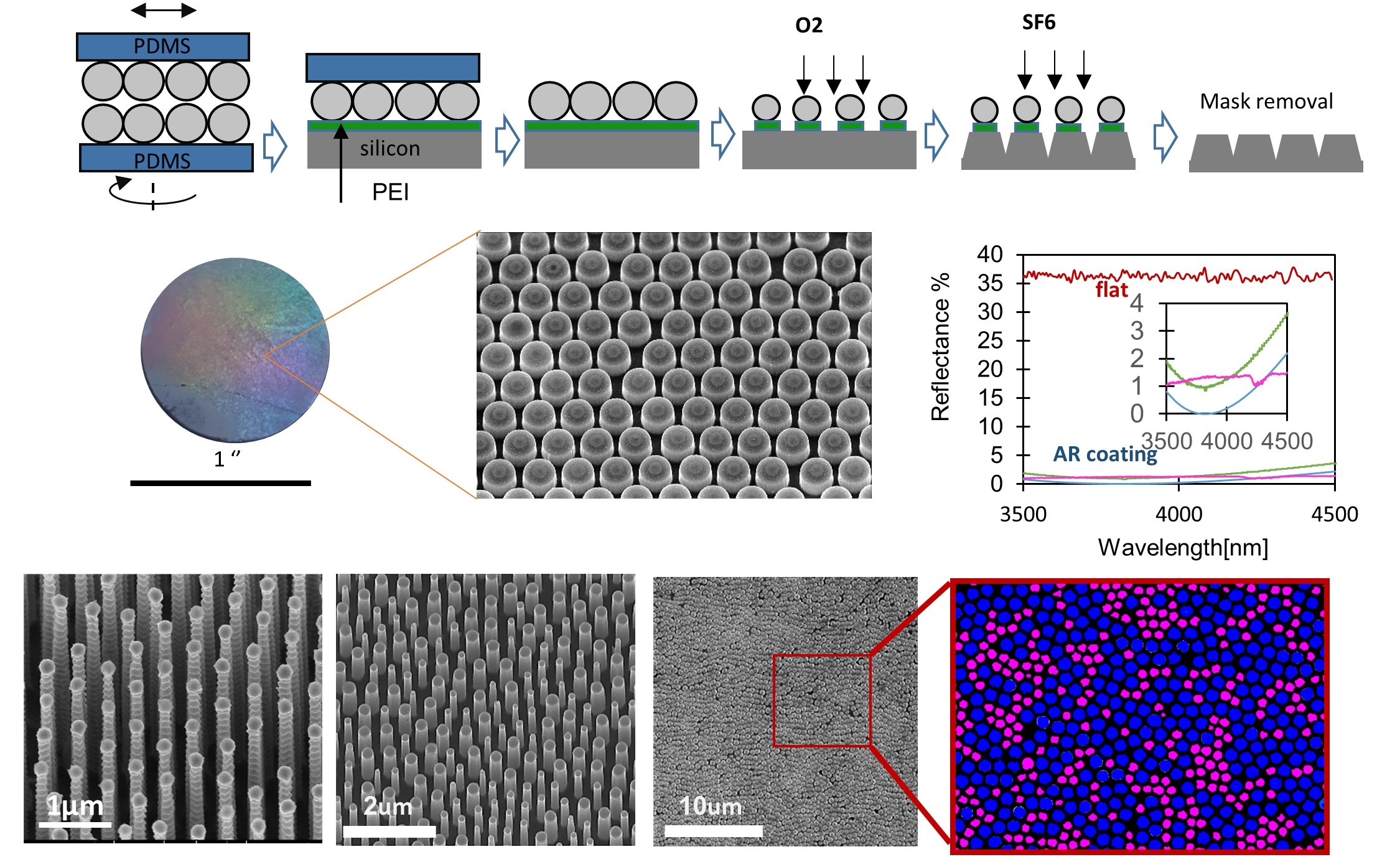
Traditional nanofabrication originates in the production of microelectronic circuits and utilizes highly expensive equipment and processes. However, today nanometric structures are widely used in areas such as optics, biomedicine, and energy harvesting, necessitating unconventional, out-of-the-box nanofabrication approaches. We are engineering novel nanofabrication methods, many of which are based on facile, cost-effective, and rapid self-assembly of synthetic building blocks.
. In contrast to commonly used self-assembly of nanoparticles from the liquid phase to the solid phase, such as dip-coating or Langmuir-Blodgett apparatus, we employ dry self-assembly. This method involves rubbing nanoparticle powder between two elastomer surfaces to form ordered arrays of nanoparticles. Recently, we automated and optimized this assembly process and developed several methods for mechanically transferring the nanoparticle arrays from the elastomer to any target surface. We apply this approach for the scalable fabrication of functional nanomaterials, such as anti-reflective structures inspired by the eyes of moths. The versatility of this process enables us to engineer moth-eye structures tailored for various materials and ranges of wavelengths, many of which are applied in our industrial collaborations with companies such as RAFAEL and ELBIT.Read more